We provide life science companies with deeply experienced professionals who excel in the highly precise technologies that produce Biologic products. Our consultants and team leaders help companies navigate the newest frontiers of biomedical research, from inception, through particular and heavily regulated manufacturing requirements, and ultimately to market.
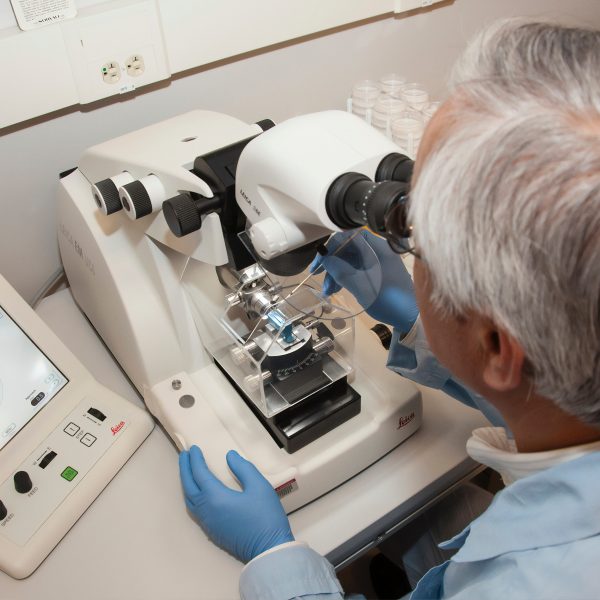
We deliver individual subject matter experts, full project teams, and project managers who provide cross-functional core team leadership. They drive stage-gated R&D processes forward and help you improve productivity, expand innovation, and reduce costs.

Our consultants rely on deep industry experience to provide effective regulatory support. We help clients respond to citations and 483 warning letters, orchestrate CAPA workstreams, calibrate acquisitions, and secure compliance.

Our SMEs and project teams drive complex clinical studies and unprecedented clinical development. We provide clinical trial physicians, clinical leaders building protocols, study management SMEs, clinical research associates, and more.

We provide project leaders with deep management experience and specific SMEs with domain expertise to drive efficiencies, tackle challenges, and realize opportunities across global manufacturing and supply chains.

We help you prove value and operationalize KOL insights by providing technology implementation, project teams, and therapeutic area SMEs such as medical science liaisons and Phase 4 medical experts.
Our SMEs have the domain expertise to handle complex programming and statistical analysis requirements. We deliver statistical programming, biostatistics, and real-world data analysis solutions that focus on timely, high-quality "fit for purpose" deliverables.
"Knowing how to match consultants with the industry knowledge who can jump right in, hit the ground running, and make it work for clients is why EG Life Sciences is a top-notch organization from top to bottom."
– Senior Project Manager and Clinical Researcher
